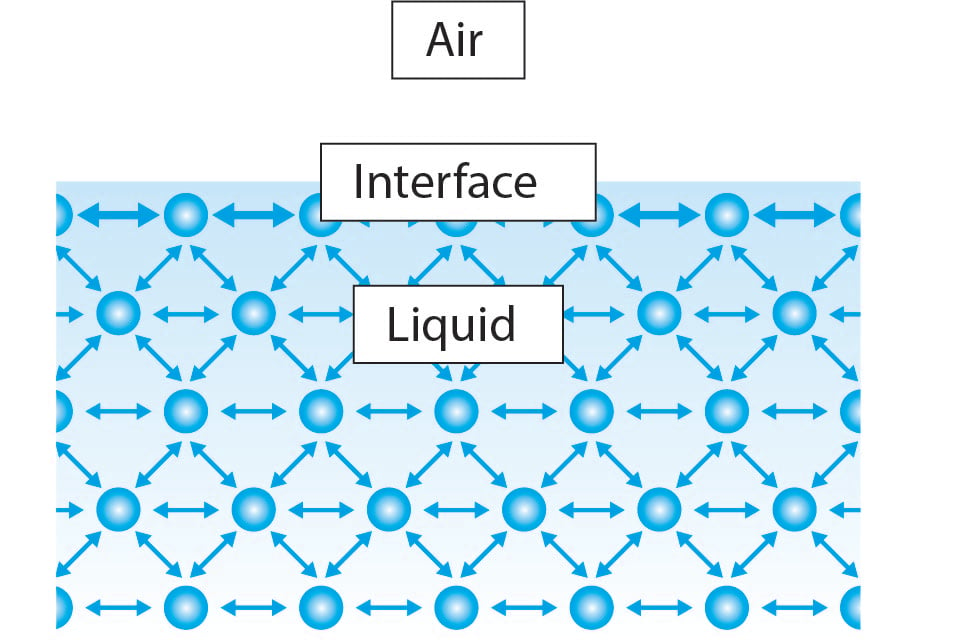
Surface Tension Of Water Glass Interface . 44 rows this is a table of surface tension values [1] for some interfaces at the indicated temperatures. surface tension is the tendency of liquid surfaces at rest to shrink into the minimum surface area possible. the answer lies in surface tension. experimental results of the surface tension of the interface between the liquid and vapor phases of ordinary water and. in this paper, we study drop spreading phenomena on a glass surface experimentally. for the water surface, or more exactly its interface with air at ambient conditions, \(\gamma \approx 0.073 \mathrm{~j} / \mathrm{m}^{2}\) (i.e. the measured value of the surface energy for water/air interface is in fact ® 1⁄4 0:073 j=m2 at room temperature.
from www.biolinscientific.com
surface tension is the tendency of liquid surfaces at rest to shrink into the minimum surface area possible. the measured value of the surface energy for water/air interface is in fact ® 1⁄4 0:073 j=m2 at room temperature. the answer lies in surface tension. for the water surface, or more exactly its interface with air at ambient conditions, \(\gamma \approx 0.073 \mathrm{~j} / \mathrm{m}^{2}\) (i.e. 44 rows this is a table of surface tension values [1] for some interfaces at the indicated temperatures. experimental results of the surface tension of the interface between the liquid and vapor phases of ordinary water and. in this paper, we study drop spreading phenomena on a glass surface experimentally.
Surface tension of water Why is it so high?
Surface Tension Of Water Glass Interface the measured value of the surface energy for water/air interface is in fact ® 1⁄4 0:073 j=m2 at room temperature. the measured value of the surface energy for water/air interface is in fact ® 1⁄4 0:073 j=m2 at room temperature. surface tension is the tendency of liquid surfaces at rest to shrink into the minimum surface area possible. experimental results of the surface tension of the interface between the liquid and vapor phases of ordinary water and. in this paper, we study drop spreading phenomena on a glass surface experimentally. 44 rows this is a table of surface tension values [1] for some interfaces at the indicated temperatures. for the water surface, or more exactly its interface with air at ambient conditions, \(\gamma \approx 0.073 \mathrm{~j} / \mathrm{m}^{2}\) (i.e. the answer lies in surface tension.
From www.atlanticgasket.com
3M Adhesion Basics / Surface Energy Atlantic Gasket Corp. Surface Tension Of Water Glass Interface 44 rows this is a table of surface tension values [1] for some interfaces at the indicated temperatures. in this paper, we study drop spreading phenomena on a glass surface experimentally. experimental results of the surface tension of the interface between the liquid and vapor phases of ordinary water and. the answer lies in surface tension.. Surface Tension Of Water Glass Interface.
From exocmokjo.blob.core.windows.net
Liquid Have Surface Tension at Janice Curry blog Surface Tension Of Water Glass Interface 44 rows this is a table of surface tension values [1] for some interfaces at the indicated temperatures. surface tension is the tendency of liquid surfaces at rest to shrink into the minimum surface area possible. in this paper, we study drop spreading phenomena on a glass surface experimentally. the measured value of the surface energy. Surface Tension Of Water Glass Interface.
From www.meritnation.com
Surface tension of water in cgs system is 72 dyne/cm What is it's value Surface Tension Of Water Glass Interface experimental results of the surface tension of the interface between the liquid and vapor phases of ordinary water and. surface tension is the tendency of liquid surfaces at rest to shrink into the minimum surface area possible. 44 rows this is a table of surface tension values [1] for some interfaces at the indicated temperatures. for. Surface Tension Of Water Glass Interface.
From stock.adobe.com
illustration of physics, Surface tension of water, the cohesive forces Surface Tension Of Water Glass Interface in this paper, we study drop spreading phenomena on a glass surface experimentally. the answer lies in surface tension. for the water surface, or more exactly its interface with air at ambient conditions, \(\gamma \approx 0.073 \mathrm{~j} / \mathrm{m}^{2}\) (i.e. 44 rows this is a table of surface tension values [1] for some interfaces at the. Surface Tension Of Water Glass Interface.
From www.science-sparks.com
Surface Tension of Water Science Experiments for Kids Surface Tension Of Water Glass Interface the measured value of the surface energy for water/air interface is in fact ® 1⁄4 0:073 j=m2 at room temperature. in this paper, we study drop spreading phenomena on a glass surface experimentally. 44 rows this is a table of surface tension values [1] for some interfaces at the indicated temperatures. surface tension is the tendency. Surface Tension Of Water Glass Interface.
From www.youtube.com
Surface tension test a must in preparing varnished glass for Surface Tension Of Water Glass Interface for the water surface, or more exactly its interface with air at ambient conditions, \(\gamma \approx 0.073 \mathrm{~j} / \mathrm{m}^{2}\) (i.e. surface tension is the tendency of liquid surfaces at rest to shrink into the minimum surface area possible. the answer lies in surface tension. experimental results of the surface tension of the interface between the. Surface Tension Of Water Glass Interface.
From bio.libretexts.org
4.5.1.3 CohesionTension Theory Biology LibreTexts Surface Tension Of Water Glass Interface experimental results of the surface tension of the interface between the liquid and vapor phases of ordinary water and. 44 rows this is a table of surface tension values [1] for some interfaces at the indicated temperatures. the measured value of the surface energy for water/air interface is in fact ® 1⁄4 0:073 j=m2 at room temperature.. Surface Tension Of Water Glass Interface.
From techblog.ctgclean.com
What is Surface Tension? CTG Technical Blog Surface Tension Of Water Glass Interface for the water surface, or more exactly its interface with air at ambient conditions, \(\gamma \approx 0.073 \mathrm{~j} / \mathrm{m}^{2}\) (i.e. the answer lies in surface tension. in this paper, we study drop spreading phenomena on a glass surface experimentally. 44 rows this is a table of surface tension values [1] for some interfaces at the. Surface Tension Of Water Glass Interface.
From www.vrogue.co
Surface Tension Part 1 Lecture 1 3 Chemical Engineeri vrogue.co Surface Tension Of Water Glass Interface 44 rows this is a table of surface tension values [1] for some interfaces at the indicated temperatures. experimental results of the surface tension of the interface between the liquid and vapor phases of ordinary water and. in this paper, we study drop spreading phenomena on a glass surface experimentally. for the water surface, or more. Surface Tension Of Water Glass Interface.
From classnotes.org.in
Surface Tension Chemistry, Class 11, States of Matter Surface Tension Of Water Glass Interface the measured value of the surface energy for water/air interface is in fact ® 1⁄4 0:073 j=m2 at room temperature. the answer lies in surface tension. for the water surface, or more exactly its interface with air at ambient conditions, \(\gamma \approx 0.073 \mathrm{~j} / \mathrm{m}^{2}\) (i.e. in this paper, we study drop spreading phenomena on. Surface Tension Of Water Glass Interface.
From chem.libretexts.org
Surface Tension Chemistry LibreTexts Surface Tension Of Water Glass Interface experimental results of the surface tension of the interface between the liquid and vapor phases of ordinary water and. for the water surface, or more exactly its interface with air at ambient conditions, \(\gamma \approx 0.073 \mathrm{~j} / \mathrm{m}^{2}\) (i.e. the answer lies in surface tension. the measured value of the surface energy for water/air interface. Surface Tension Of Water Glass Interface.
From www.biolinscientific.com
3 ways to measure surface tension Surface Tension Of Water Glass Interface for the water surface, or more exactly its interface with air at ambient conditions, \(\gamma \approx 0.073 \mathrm{~j} / \mathrm{m}^{2}\) (i.e. the measured value of the surface energy for water/air interface is in fact ® 1⁄4 0:073 j=m2 at room temperature. in this paper, we study drop spreading phenomena on a glass surface experimentally. surface tension. Surface Tension Of Water Glass Interface.
From www.flickr.com
Surface Tension A glass of water about to spill. That is t… Flickr Surface Tension Of Water Glass Interface experimental results of the surface tension of the interface between the liquid and vapor phases of ordinary water and. 44 rows this is a table of surface tension values [1] for some interfaces at the indicated temperatures. the answer lies in surface tension. in this paper, we study drop spreading phenomena on a glass surface experimentally.. Surface Tension Of Water Glass Interface.
From chemwiki.ucdavis.edu
Surface Tension Chemwiki Surface Tension Of Water Glass Interface the measured value of the surface energy for water/air interface is in fact ® 1⁄4 0:073 j=m2 at room temperature. the answer lies in surface tension. experimental results of the surface tension of the interface between the liquid and vapor phases of ordinary water and. surface tension is the tendency of liquid surfaces at rest to. Surface Tension Of Water Glass Interface.
From www.researchgate.net
Schematic illustration of standard methods of surface tension Surface Tension Of Water Glass Interface in this paper, we study drop spreading phenomena on a glass surface experimentally. the measured value of the surface energy for water/air interface is in fact ® 1⁄4 0:073 j=m2 at room temperature. the answer lies in surface tension. surface tension is the tendency of liquid surfaces at rest to shrink into the minimum surface area. Surface Tension Of Water Glass Interface.
From www.youtube.com
Surface Tension of Water (Mechanics lab.) YouTube Surface Tension Of Water Glass Interface surface tension is the tendency of liquid surfaces at rest to shrink into the minimum surface area possible. 44 rows this is a table of surface tension values [1] for some interfaces at the indicated temperatures. the measured value of the surface energy for water/air interface is in fact ® 1⁄4 0:073 j=m2 at room temperature. . Surface Tension Of Water Glass Interface.
From www.youtube.com
The Sci Guys Science at Home SE3 EP4 Water Glass Surface Tension Surface Tension Of Water Glass Interface for the water surface, or more exactly its interface with air at ambient conditions, \(\gamma \approx 0.073 \mathrm{~j} / \mathrm{m}^{2}\) (i.e. surface tension is the tendency of liquid surfaces at rest to shrink into the minimum surface area possible. experimental results of the surface tension of the interface between the liquid and vapor phases of ordinary water. Surface Tension Of Water Glass Interface.
From funsizephysics.com
What is Surface Tension? FunsizePhysics Surface Tension Of Water Glass Interface for the water surface, or more exactly its interface with air at ambient conditions, \(\gamma \approx 0.073 \mathrm{~j} / \mathrm{m}^{2}\) (i.e. in this paper, we study drop spreading phenomena on a glass surface experimentally. the measured value of the surface energy for water/air interface is in fact ® 1⁄4 0:073 j=m2 at room temperature. experimental results. Surface Tension Of Water Glass Interface.